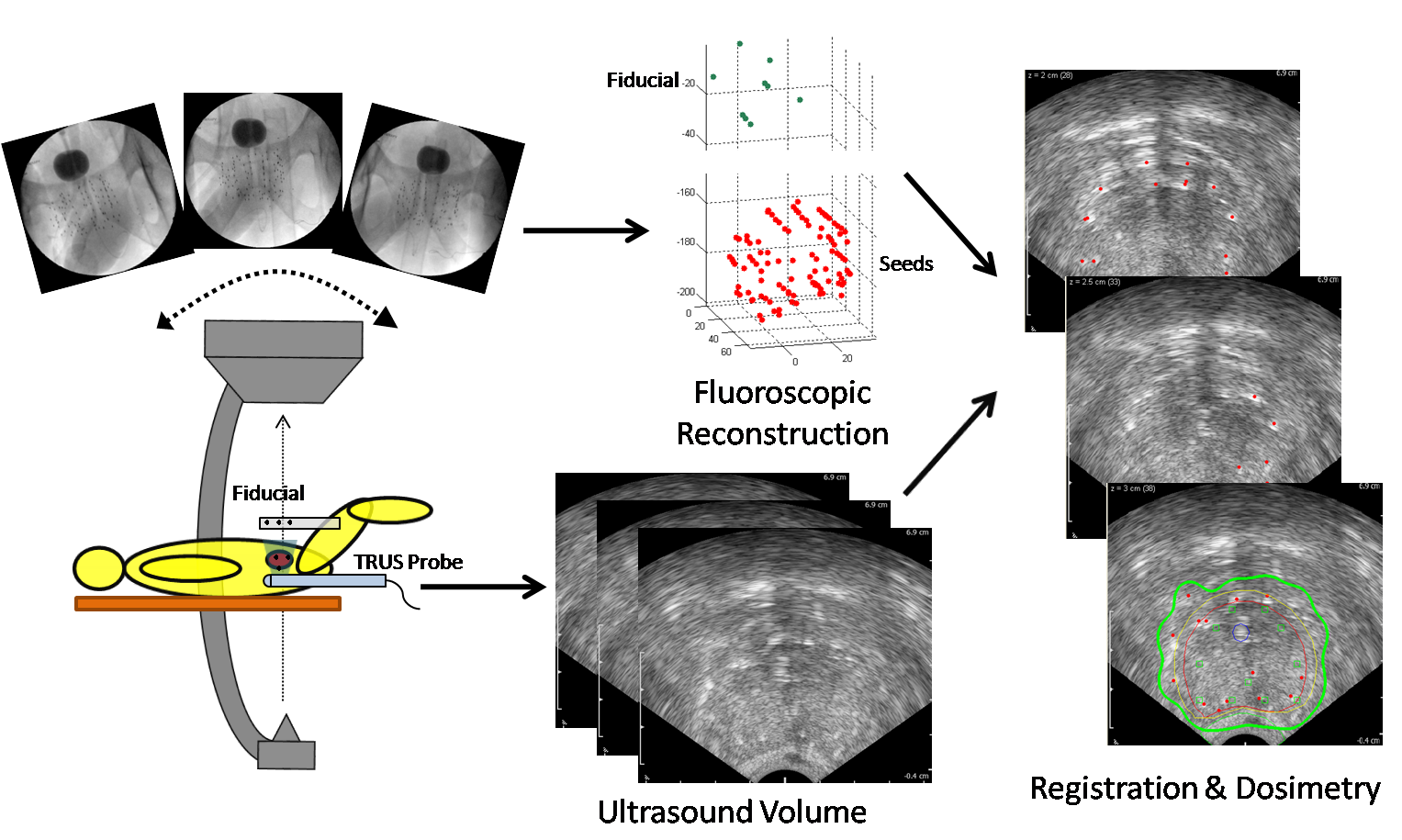
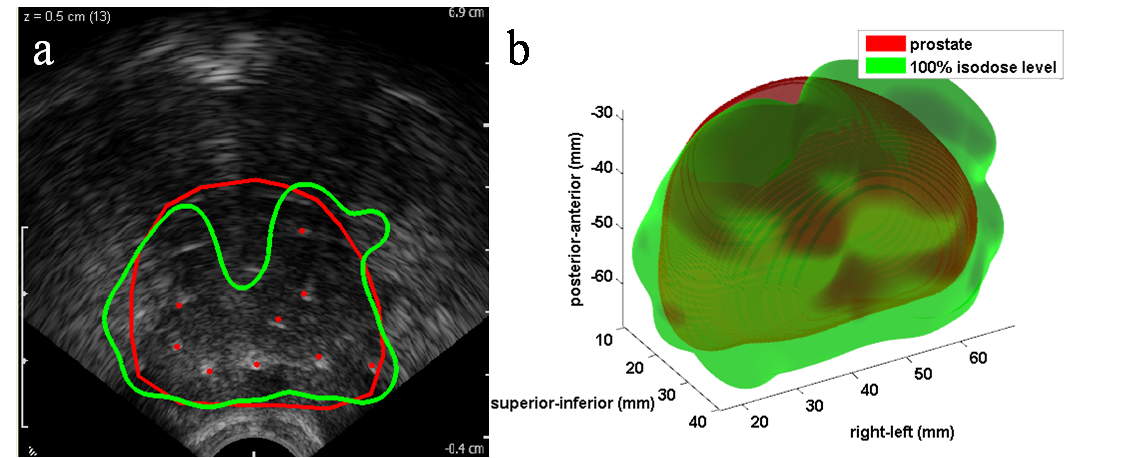
Intraoperative Image-Guidance System for Dynamic Dosimetry in Prostate Brachytherapy Introduction Prostate cancer is diagnosed in approximately 192,000 men per year in the United States, making it the most common cancer afflicting men and second most common in the overall population. Brachytherapy (implantation of radioactive seeds into the prostate) is widely used in the treatment of prostate cancer, yet studies show that it is common for men to receive suboptimal brachytherapy treatment, with doses either insufficient (leading to risk of cancer recurrence) or too high (leading to side effects and sometimes serious toxicity). A major contributor to these outcomes is the lack of existing techniques for physicians to visualize or 'see' radiation dose within the prostate as seeds are implanted; the goal of the proposed research is to develop a system which achieves this capability in a manner that can be readily implemented in operating rooms on a widespread scale nationwide. Brachytherapy is widely utilized in the management of prostate cancer; over 55,000 procedures are performed in the US annually. The prevalence of brachytherapy is due to its minimally invasive nature and modest time commitment on the part of the patient. Its success hinges on adequately dosing the prostate while avoiding excessive radiation to adjacent organs. Despite some improvements in technique and excellent published results from select centers, contemporary multi-center reports and quality assurance programs document considerable variability in results, with ensuing toxicity and suboptimal outcomes. Well-recognized major contributors to poor dosimetric results are source positioning errors, poor source visualization, and prostatic edema, which in aggregate lead to both regions of excessive as well as inadequate dose. The critical need for dynamic visualization of sources and intraoperative dose reconstruction during the procedure is broadly recognized. Intraoperative dose optimization promises to dramatically change the standard of care in brachytherapy by allowing the physician to precisely tailor the dose to the individual patient's anatomy, as well as obtain accurate dose visualization and feedback prior to procedure completion. This requires intra-operative localization of the implant in relation to the prostate and surrounding anatomy. We propose the development of a clinically feasible image-guidance platform with capability for dynamic, precise and quantitative evaluation of dose during brachytherapy. This will allow for ongoing correction for source position alterations and result in an optimal implant, leading to reduced toxicity and improved cancer control. Aim 1: Development of Dynamic Dosimetry: Develop a system concept and algorithms for intraoperative dosimetry and implant optimization. The result will be a software-based, practical method for true intra-operative dose visualization. The system will not require significant changes in clinical hardware and will minimally alter customary clinical workflow, making it feasible for translation into widespread use. Aim 2: System Implementation and Validation: Perform technical implementation of the system components into a brachytherapy dosimetry suite suitable for clinical use and evaluation of the dynamic dosimetry system intraoperatively. Full documentation and verification of its technical accuracy and robustness in phantoms will be conducted, followed by pilot clinical studies to validate the system and assess performance and suitability for intraoperative use. This information will be fed into system updates for Aim 3. Aim 3: Clinical Hypothesis and Trial: Perform IRB-approved trials utilizing individual system components, as well as a final study of the fully integrated system, in order to establish performance against a rigorous set of benchmarks and to compare with currently available standard methods. Estimates of outcomes will be obtained for confirmation in a future, larger Phase II study. An Image-Guidance System for Dynamic Dosimetry Our proposed system fuses transrectal ultrasound (TRUS) and X-ray fluoroscopy based on the mobile C-arm. Three or more distinct 2D fluoroscopic images are taken of the seeds and a simple fiducial with seed-like markers, generally within a 20° cone of the anterior-posterior axis of the patient lying on the table. TRUS images are taken to acquire the prostate volume. Both image sets are processed through the following steps: 1) seed segmentation, 2) fiducial detection with pose estimation, 3) seed matching with reconstruction, and 4) fluoroscopy-to-TRUS registration (see Fig. 1). Combining these algorithms, we have an image-guidance system which localizes implanted seeds for dynamic dosimetry. A straightforward computation based on seed activity information and these registered seed coordinates results in our final desired dose calculation. The system as a whole also has the capability of detecting cold spots (see Fig. 2). Fig. 1. System workflow of our image-guidance system for dynamic dosimetry. Fig. 2. Intraoperative dosimetry result showing a cold spot. (a) TRUS image is overlaid with prostate contour (thick red line) and the 100% isodose level (thick green line) computed from the registered seed reconstruction (red dots). (b) 3D rendering of the same prostate and 100% isodose level; cold spot is evident at the anterior-superior end of the prostate. |
|
|